Abstract
Background: Hairy cell leukemia-variant (HCL-v) is one type of chronic lymphocytic proliferative disorders which was classified into splenic B-cell lymphoma/leukemia, unclassifiable. Both clinical and laboratory characteristics and treatment strategy remain elusive due to the rarity of the disease. Here, we firstly presented the diseases features and efficacy of a variety of treatment options in a large cohort from China.
Methods: Thirty-three patients were diagnosed with HCL-v in Institute of Hematology & Blood Diseases Hospital, Chinese Academy of Medical Science & Peking Union Medical College from June 1990 to February 2021. We analyzed the disease characteristics and treatment outcome, especially in terms of clinical manifestation, immunophenotypic and molecular evaluation and efficacy of multiple first-line treatment.
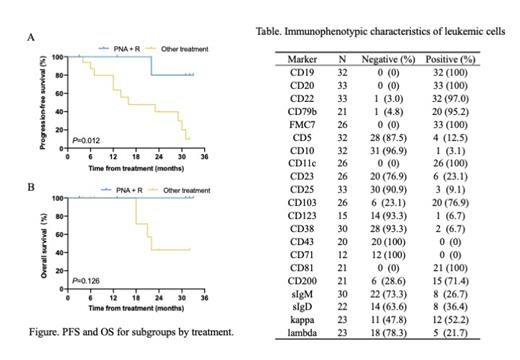
Results: The median age of this cohort was 59 years (range, 34-79 years) at diagnosis, with 23 males and 10 females. Abdominal mass and relative signs (n=22) and abnormal complete blood count (n=9) were the most common chief complaints. Splenomegaly was present in 32 (97.0%) cases, among whom 26 (78.8%) cases were massive. Leukocytosis and leukopenia were presented in 23 (69.7%) patients and 5 (15.2%) patients, with a median white blood cell count of 21.58×10 9/L (range, 1.34-224.59×10 9/L). The median percentage of the leukemic cells in bone marrow tested by flow cytometry was 33% (range, 6.2%-96%), and immunophenotyping showed monoclonal B-cells positive for pan B-cell antigens (CD19, CD20, CD22, CD79b) in almost all patients (Table). CD11c was positive in all patients, and CD103 was positive in 20/26 (76.9%) patients. CD25 was negative in 30/33 (90.9%) patients, and CD23 was negative in 20/26 (76.9%) cases. CD200 was frequently expressed among patients (15/21, 71.4%). For the pathological pattern of bone marrow involvement, 20/27 (74.1%) patients showed a predominantly interstitial pattern. Moreover, Annexin A1 was negative in all (n=12) patients detected by immunohistochemistry. Conventional cytogenetic analysis showed abnormal karyotype in 10/24 (41.7%) patients, including 6 patients with complex karyotype. Fluorescence in situ hybridization analysis showed deletion of TP53 in 4/20 (20.0%) patients and IGH translocation in 5/16 (31.3%) patients. The BRAF V600E mutation was negative in all the patients (n=20). In 2 of 14 (16.7%) patients, TP53 mutation was detected by next-generation sequencing. Sixteen of nineteen (84.2%) patients showed monoclonal IGHV rearrangements, and the most common rearrangement fragment was VH4-34 (n=3, 18.8%). Six of seventeen (35.3%) patients presented with unmutated IGHV and patients with VH4-34 were all unmutated. 31 patients needed treatment finally. Treatment choices included interferon-α (IFN-α) in 11 patients, chlorambucil (CLB) in 5 patients, single purine nucleoside analogs (PNA) in 3 patients and PNA plus rituximab in 9 patients. Four patients who received IFN-α or CLB treatment also underwent splenectomy. The objective response rate was 100% for patients receiving PNA plus rituximab and 77% for cases receiving others. And people treated with PNA plus rituximab had a higher complete response rate compared to the other regimen (75% versus 12%, P = 0.004). During a median follow-up of 32 months (range, 3-207) for 29 patients, 14 (48.3%) patients experienced at least one relapse or progression, and 7 (24.1%) patients had 2 or more relapses. Of note, one case also underwent a diffuse large B-cell lymphoma transformation. The median PFS and OS were 31 months (95% CI 25.5-36.5) and 70 months (95% CI 50.5-89.5), respectively. PNA plus rituximab prolong PFS compared to the others (3-year PFS rate 80% [95%CI 20-97] versus 10% [95%CI 1-35], P = 0.012, Figure A). But no significant difference in 3-year OS rate was observed between two groups (100% [95%CI 100-100] versus 43% [95%CI 10-73], P = 0.128, Figure B).
Conclusion: HCL-v is a rare disease with specific clinical and immunophenotypic features but may overlap with classic hairy cell leukemia or other splenic B-cell neoplasms. Overall, it is an indolent lymphoma with recurrent progression, and the use of PNA plus rituximab in first-line treatment can result in a deeper and longer remission.
Wang: AbbVie: Consultancy; Astellas Pharma, Inc.: Research Funding.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal